Covid-19, EU – Baltic States, Good for Business, Legislation, Markets and Companies, Medicine, USA
International Internet Magazine. Baltic States news & analytics
Sunday, 21.12.2025, 10:42
EU approves contract with Moderna for supply of coronavirus vaccines
 Print version
Print version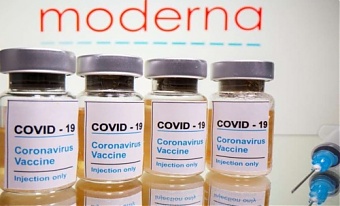 |
|---|
Liepina said that the contract provides for the initial purchase of 80 million doses on behalf of all EU member states, plus an option to request up to a further 80 million doses, to be supplied once a vaccine has proven to be safe and effective against Covid-19.
The contract with Moderna will enlarge the already broad portfolio of vaccines to be produced in Europe, including the contracts signed with AstraZeneca, Sanofi-GSK, Janssen Pharmaceutica NV, BioNTech-Pfizer and the contract approved with CureVac.
This diversified vaccines portfolio will ensure Europe is well prepared for vaccination, once the vaccines have been proven to be safe and effective. Member states can also decide to donate the vaccine to lower and middle-income countries or to re-direct it to other European countries.
The decision to support this vaccine is based on a sound scientific assessment, the technology used, and its production capacity in Europe to supply the whole of the EU, Liepina said.
The European Commission presented on 17 June a European strategy to accelerate the development, manufacturing and deployment of effective and safe vaccines against Covid-19. In return for the right to buy a specified number of vaccine doses in a given timeframe, the Commission finances part of the upfront costs faced by vaccines producers in the form of Advance Purchase Agreements. Funding provided is considered as a down-payment on the vaccines that will actually be purchased by member states on the basis of the advance purchase agreements.
Since the high cost and high failure rate make investing in a Covid-19 vaccine a high-risk decision for vaccine developers, these agreements will therefore allow investments to be made that otherwise might not happen.
Once vaccines have been proven to be safe and effective and have been granted market authorization by the European Medicines Agency, they need to be quickly distributed and deployed across Europe. On October 15, the Commission set out the key steps that member states need to take to be fully prepared, which includes the development of national vaccination strategies. The Commission is putting in place a common reporting framework and a platform to monitor the effectiveness of national vaccine strategies.
- 28.01.2022 BONO aims at a billion!
- 26.08.2021 LLC Dizozols Investments finalizes investment attraction deal with Crowdestor with record-high profits
- 13.02.2021 Моя жизнь в газете. Очерки по новейшей истории Латвии. Глава 1
- 30.12.2020 Накануне 25-летия Балтийский курс/The Baltic Course уходит с рынка деловых СМИ
- 30.12.2020 On the verge of its 25th anniversary, The Baltic Course leaves business media market
- 30.12.2020 Business Education Plus предлагает анонсы бизнес-обучений в январе-феврале 2021 года
- 30.12.2020 Hotels showing strong interest in providing self-isolation service
- 30.12.2020 EU to buy additional 100 mln doses of coronavirus vaccine
- 30.12.2020 ЕС закупит 100 млн. дополнительных доз вакцины Biontech и Pfizer
- 29.12.2020 Latvia to impose curfew, state of emergency to be extended until February 7








 «The Baltic Course» Is Sold and Stays in Business!
«The Baltic Course» Is Sold and Stays in Business!

